Asia-Pacific Forum on Science Learning and Teaching, Volume 21, Issue 1, Article 9 (Dec., 2021) |
Apparatus
The apparatus encompasses a transparent plastic file which is not too thick and not too thin, candle (needed to separate the file into compartments as seen in Figure 1), newspaper or magazine, water, and paper glass or a cup. Carefully as shown in Figure 1 seal the plastic file with the candle flame to separate it into compartments like a U tube. Carefully seal both sides of the plastic file inside of the newspaper or magazine so that when you are asked by students to show what it is inside of the newspaper, you can show them there is nothing inside the newspaper to strengthen your claim. Now the set-up is ready to present as a lecture demonstration. Carefully pour the water into the open part of the newspaper by using a glass or a cup. The water will rise higher. Then invert the newspaper so that the water will begin to flow through the closed part of the plastic file. Invert again the newspaper in its original position so that the water will flow back to its original situation in a short time. Then you can pour back the water to the glass or the cup.
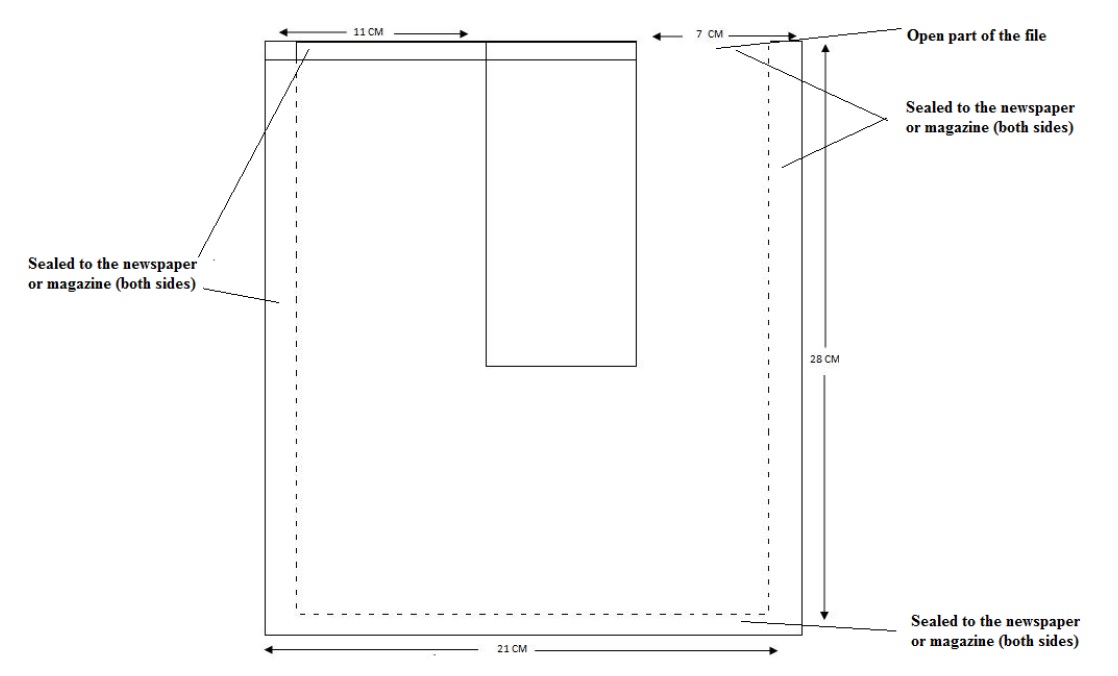
Figure 1: Transparent plastic file sealed to the newspaper/magazine
Teaching Approach
It is enlightening to explore the physics behind this demonstration. When the water is poured into newspaper! -actually into the plastic file, the water will rise higher in this U tube shaped file. The water will rise because of the surface tension and capillary action. The cohesive forces between water molecules create surface tension. Surface tension and adhesion create capillary action that adhesion of water to the walls of the file causes an upward force on the water at the edges of the file and result in a curved surface of a liquid which turns upward (Figure 2 a and b). The surface tension acts to hold the surface intact, so instead of just the edges moving upward, the whole water surface is dragged upward. Water molecules experience strong intermolecular attractive forces. When those forces are between like molecules, they are referred to as cohesive forces. When the attractive forces are between unlike molecules, they are called as adhesive forces. The water molecules at the surface do not have other like molecules on all sides of them and consequently they cohere more strongly to those directly associated with them on the surface. The adhesive forces between water molecules and the walls of the plastic file are stronger than the cohesive forces lead to an upward turning meniscus at the walls of the plastic file and contribute to capillary action.
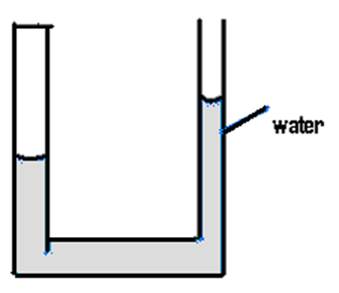
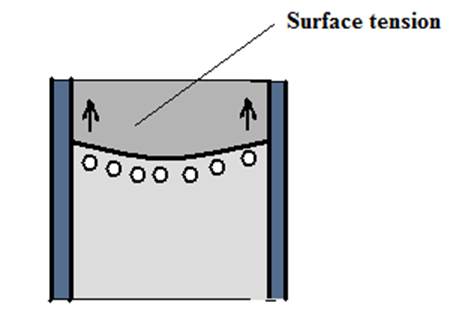
Figure 2 (a) and (b): Surface tension and capillary action
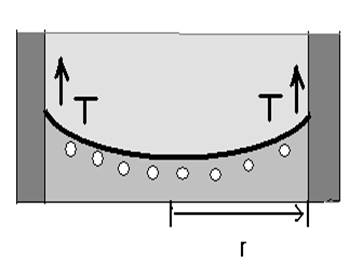
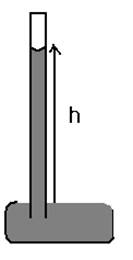
Fupward = T*2πrT: surface tension
ρ: density of liquid (in this case, water)
T*2πr= ρg(hπr2)
h = 2T/(ρrg)h: the height of the liquid can be lifted by capillary action
Figure 3 (a) and (b): Calculation of capillary action
Figure 3 (a) and (b) shows calculation of capillary action which is the height to which capillary action will take water in a circular tube. Acting around the circumference, the upward force is Fupward = T*2πr. We can find h as shown in Figure 3 (b) as follows:
Fupward = Fg⇒ T*2πr = mg
m = ρv ⇒ m = ρ(hπr2)
T*2πr = ρ(hπr2) g ⇒ h = 2T/(ρrg)
g: gravity
v: volume of tube (cylinder)
Copyright (C) 2021 EdUHK APFSLT. Volume 21, Issue 1, Article 9 (Dec., 2021). All Rights Reserved.