Asia-Pacific Forum on Science Learning and Teaching, Volume 17, Issue 1, Article 9 (Jun., 2016) |
Appendix A
Sample Handout Problem for the Groups
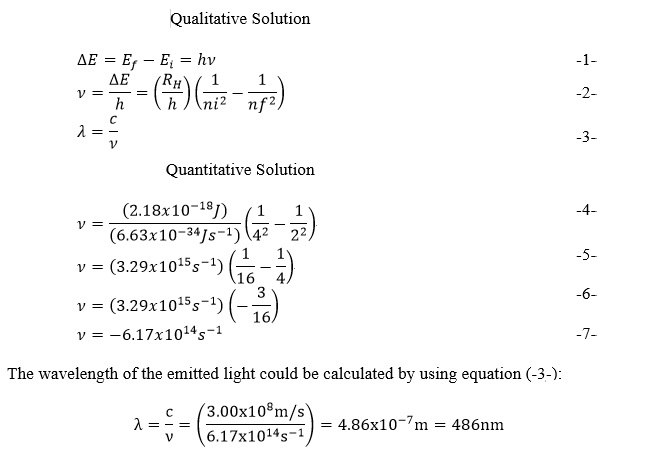
“Calculate the wavelength of light that corresponds to the transition of the electron from the n=4 to n=2 state of the hydrogen atom. Is the light absorbed or emitted” (Brown et al., 2000)?
(IFP): “What is (are) the fundamental principle(s)/concept(s) of the problem”?
(SLV): “Which equation(s) do you need to solve the problem? What is the correct answer to the problem”?
(CHK): “What are the unit, sign, and magnitude of the asked variables(s)”?Solution steps
IFP- “First of all, identifying fundamental principle(s)/concept(s) of the problem is determined by the students. Then the concepts, known, unknown variables, and constants are indicated. If it is necessary, the problem can be visualized with the help of simple diagram/chart”.
Principles- The wave nature of light; quantized energy; Bohr’s model of the hydrogen atom
Concepts- Energy level; Rydberg and Planck constants; the principal quantum numbers of the initial and final states of the atom; frequency and wavelength of light.Constants: RH= 2.18×10-18 J; h=6.63×10-34 Js-1; c=3.00 ×108m/s
Known variables: ni=4; nf=2
Unknown variables: y; λSLV- “Secondly, the problem is solved qualitatively and quantitatively. Qualitative solutions are performed with the help of required equations. A mathematical model is established for finding desired unknown variable. The desired unknown variable is calculated by using the given variables in this section”.
CHK- “Finally, the unit, sign, and magnitude of the variable are checked in this section”.The negative sign indicates that light with a frequency of is emitted. 486 nm is the wavelength of the green emission line in the spectrum of hydrogen.