Asia-Pacific Forum on Science Learning and Teaching, Volume 16, Issue 2, Article 4 (Dec., 2015) |
Appendix A: Examples of the items in SMAT
Item 3: Which of the followings is false about evaporation? (developed by the researchers)
A) Evaporation begins after a substance is heated until its boiling point. *
B) Evaporation occur at all temperatures.
C) The vapor pressure of a liquid depends on its molecular structure and the temperature.
D) The vapor pressure of a liquid has the lowest value at freezing point of liquid.
E) If the attractive forces between molecules of a liquid are less, the liquid evaporates more easily.
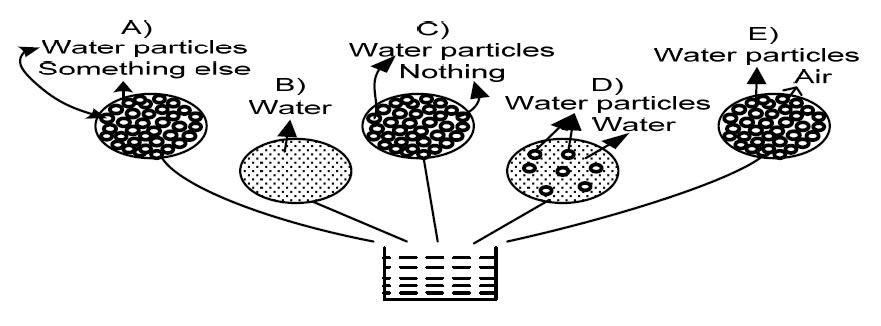
Item 10. Your picture of pure water in its finest detail, would be most like which of the following drawings? (adapted from Osborne and Freyberg, 1985).
Item 22: In the summer, smokes rises from the soil after rain. What is the reason of this event occurring? Please explain.
…………………………………………………………………………………………..
Item 24: When you compare the states of matter (solid, liquid and gas) in terms of weight what would you say? (in a closed container). Please explain.
……………………………………………………………………
Appendix B: Activity 1: What are all substances mainly made of? (Translated from Turkish)
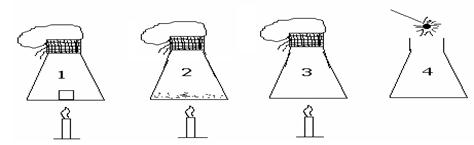
Name and Surname: Group name:In the following figure, an ice cube is in pan K, balanced by weights in the other pan. After a period of time, the ice melted completely. When the ice in the pan K melts, what happens to the balance?
Write your answer
………………………………………
………………………………………
………………………………………
………………………………………
……………………………...........
Now, let’s perform the following activity.
Materials:
One small balloon, one small flask, one ice cube, a match, a piece of string, hot plate, balance, oven mittProcedure:
1. Weigh the flask, ice cube, and balloon using the balance. Record the weight on the following data table.
2. Stretch the open balloon over the top of the flask and tie it with a string.
3. Heat the flask until the ice cube melts. Write down your observations of the ice cube and the balloon on the data table.
4. Using an oven mitt, place the bottle with balloon on the balance; record the weight on the data table.
5. Heat the flask until the water evaporates completely. Write down your observations of the water and the balloon on the data table.
6. Using an oven mitt, place the flask with balloon on the balance; record the weight on the data table.
7. Quickly hold the burning match into the mouth of the flask.
Write down your observations of the burning match on the data table.
Data table
Weight (gram)
Observations
Flask, balloon, ice cube
Flask, liquid, balloon
Flash, water vapor, balloon
Experience of the burning match
Questions:
1. What do you think caused the balloon to expand?
2. If water changes states from liquid to gas to solid, how does its mass change?
3. If water changes states from liquid to gas to solid, how does the size of its molecules change?
4. If water changes states from liquid to gas to solid, how do interactions between molecules change?