Asia-Pacific Forum on Science Learning and Teaching, Volume 17, Issue 2, Article 18 (Dec., 2016) |
The flow maps derived for each prospective chemistry teacher (P) were analysed according to the mentioned quantitative variables in this study. The results obtained are shown in Table 1.
Table 1: The cognitive structure outcomes of prospective chemistry teachers
Variables
P1
P2
P3
P4
P5
P6
P7
P8
Extent
11
10
9
13
12
12
12
15
Richness
5
5
5
8
8
8
8
10
Integratedness
0.31
0.33
0.35
0.38
0.4
0.4
0.4
0.4
Accuracy
1
1
0
0
0
0
0
0
A close examination of Table 1 makes it clear that the number of linear linkages providing information concerning the extent of knowledge that prospective chemistry teachers remembered ranges between 9 and 15, whereas the number of linkages providing information on the richness of knowledge linkages ranges between 5 and 10. Accordingly, we see that the variable of integratedness receives values between 0.31 and 0.4, and that the number of misconceptions (accuracy) is 1 at most. The misconceptions determined are shown in Table 2.
Table 2: Misconceptions determined
Misconceptions
- Oxidation state is the number of electrons an ion has.
- Oxidation state is the stage at which an element gains electrons.
Also, the flow maps of P8, P4 and P1 are examined in detail. The flow map formed from the written narrative of P8 is shown in Figure 1.

Figure 1
According to Figure 1, the number of total ideas (the number of linear linkages) in the flow map for P8 is 15, and the number of recurrent linkages is 10. On examining the recurrent linkages, it is found that item 5, for instance, “oxidiser is reduced”, is linked to item 3, “oxidation-reduction reactions are based on losing and gaining electrons”, with a recurrent arrow because it contains reduction which was mentioned before. In the same way, item 6, “oxidiser oxidises other substances”, is linked to item 4, “oxidiser gains electrons”, with a recurrent arrow since it contains the previously mentioned expression oxidiser. Item 4, “oxidiser gains electrons”, is linked to item 3, “oxidation-reduction reactions are based on losing and gaining electrons”, with a recurrent arrow since it contains the previously mentioned expression, gaining electrons. Figure 2 shows the flow map formed from the written narrative of P4.
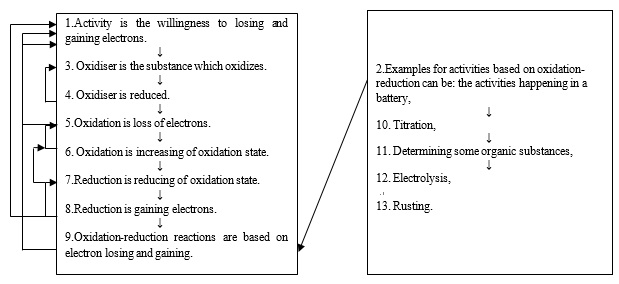
Figure 2: The flow map formed from the written narrative of P4.According to Figure 2, the number of total ideas (the number of linear linkages) in the flow map for P4 is 13, and the number of recurrent linkages is 8. On examining the recurrent linkages, it is found that item 8, for instance, “Reduction is gaining electrons” is linked to item 1, “Activity is the willingness to losing and gaining electrons” with a recurrent arrow because it contains gaining electrons which was mentioned before. In the same way, item 9 “Oxidation-reduction reactions are based on electron losing and gaining” is linked to item 1 “Activity is the willingness to losing and gaining electrons” with a recurrent arrow since it contains the previously mentioned expression losing and gaining electrons. Figure 3 shows the flow map formed from the written narrative of P1.
* Misconception
Figure 3: The flow map formed from the written narrative of P1. As is clear from Figure 3, the number of total ideas (the number of linear linkages) in the flow map for P1 is 11, while the number of recurrent linkages is 5, and the number of misconceptions is 1. On examining the recurrent linkages, it is found that item 4, for instance, “oxidiser gains electrons and oxidises other substances”, is linked to item 3, “reduction is gain of electrons”, with a repeated arrow because it contains the previously mentioned expression electron gaining. Similarly, item 7 “oxidation-reduction reactions are the reactions in which oxidation and reduction occur between two species”, is linked to item 1, “oxidation is loss of electrons”, because it contains the word oxidation, and to item 3, “reduction is gain of electrons”, with a recurrent arrow because they contain the statement, reduction. Also, item 6 in the flow map, “oxidation state is the number of electrons an ion has”, is a misconception.