Asia-Pacific Forum on Science Learning and Teaching, Volume 17, Issue 2, Article 5 (Dec., 2016) |
Activity 1: An analysis of redox reactions
(How does electron exchange happen during oxidation and reduction?)
Model 1: Oxidation and Reduction
Critical Thinking Questions
- How did Cu1+ change in the direction submarine gives out water?
- How did Cu1+ change in the direction submarine takes in water?
- In which direction was there an increase in Cu1+?
- How did oxidation happen in the valence of copper (by releasing electrons or by attracting electrons?)
- How did reduction happen in the valence of copper (by releasing electrons or by attracting electrons?)
- Write down the relationship of electrons with oxidation and reduction.
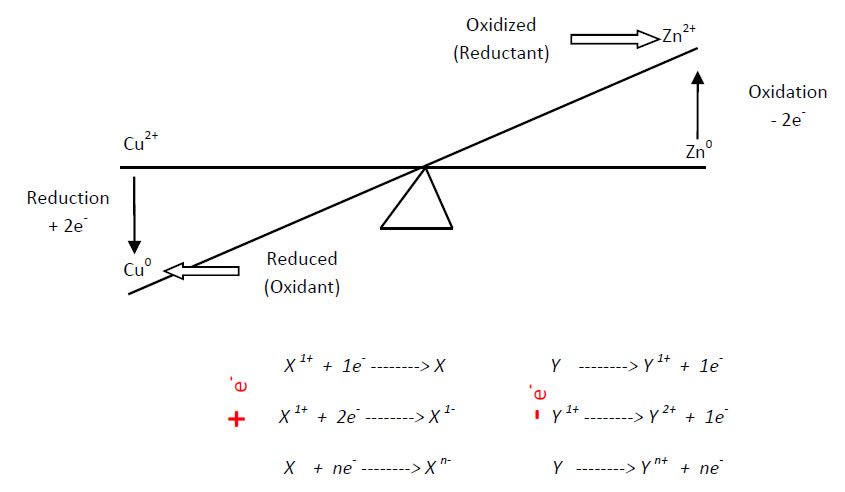
Model 2: Oxidation, Reduction, and Electron Exchange
Critical Thinking Questions
- In which element did oxidation happen?
- In which element did reduction happen?
- Which is the oxidized element?
- Which is the reduced element?
- What do we call the substance oxidizing the element opposite when it attracts electrons?
- What do we call the substance reducing the element opposite when it releases electrons?
- Which is oxidant and which is reductant?
- Study the X and Y reactions in the model, and write down the reaction where the Zn0 element releases 2 electrons.
- Write down the reaction where Cu2+ attracts 2 electrons.
Copyright (C) 2016 EdUHK APFSLT. Volume 17, Issue 2, Article 5 (Dec., 2016). All Rights Reserved.