Asia-Pacific Forum on Science Learning and Teaching, Volume 16, Issue 2, Article 11 (Dec., 2015) |
The PBL scenario, whose details are presented below, has been designed to be appropriate to the science teachers' class level. It can be used at the high school level as well, as long as a few changes are made. The scripts in the “I'm So Cold” scenario are presented below consecutively.
SESSION 1
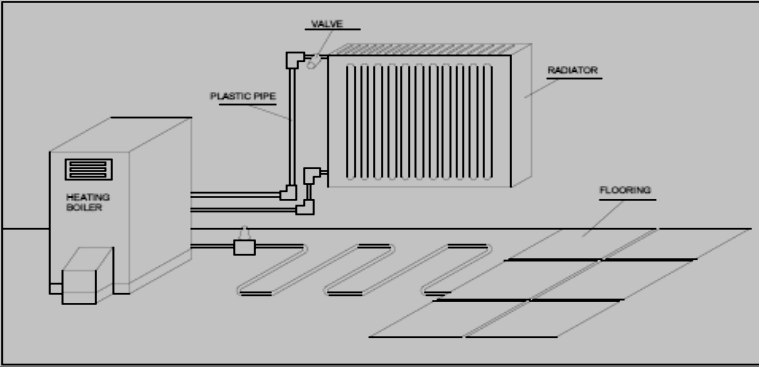
Part 1Mr. Şahin and his family have moved to a new house. A heating system is installed in the house before they move in. With the coming of winter, the house needs to be heated. So the family wants the heating system to be turned on so that they can meet their heating needs. About 25 minutes after the radiators are turned on and the house begins to be heated up, they suddenly realize that the house is not getting any warmer. When they check the boiler on their balcony, they find that the water has been depleted and the heating system has automatically stopped because of this for safety (The heating system of the house is shown in Figure 1).
- What is the problem here?
- There is a problem with heating the house. After the system is turned on, the radiators no longer give out any heat.
- Write down your hypothesis about the source of the problem.
- There might be a problem with the connections in the plumbing in the house.
- There might be a problem with the mechanical installations of the heating system.
- The water in the radiators might have evaporated.
- The inner parts of the heating system (the parts of the installation where pipes cannot be seen) might have a water seepage somewhere.
- What can be done to solve this problem?
- The plumbing of the house (the connections in the heating system) can be checked.
- The mechanical installations of the heating system can be checked.
- Some technical information can be found about the heating system (the amount of water in the boiler, heat efficiency, etc.).
- The infrastructure of the heater can be examined and whether or not there is a water seepage somewhere in the plumbing can be checked, and if there is, this leakage can be closed off.
Figure 1. A Simple Diagram of the Heating System
SESSION 1
Part 2Mr. Şahin calls in the thermodynamic authorized service and explains the problem to the technicians so that the source of the problem can be determined and solved. The technicians from the authorized service check the system and explain that there is no problem with the mechanical installation of the heater and also no problem with the plumbing in the house or the connections in the heating system installations. The technicians dwell on the fact that there is a significant reduction in the level of water in the boiler.
- Please summarize the information provided.
- It was determined that there was no problem in the connections between the house plumbing and the heating system.
- The work of the technical service technicians who came to examine the problem showed that there was no problem in the mechanical equipment of the heating system.
- Compare what you know now with your hypotheses.
- There might be a problem with the connections in the plumbing in the house. (eliminated)
- There might be a problem with the mechanics of the heating system. (eliminated)
- The water in the radiators might have evaporated.
- There might be water seepage in the parts of the installation where the pipes are hidden.
- Discuss the physical mechanism of a reduction in the water level of the heating system boiler (in terms of physical laws and principles).
- Water evaporation
- Heat exchange of matter
SESSION 1
Part 3The service personnel think that the heat resulting from when the heating system is in operation (fuel is consumed), causes the water to evaporate and a steady decrease in the water occurs and stops the system (it is believed that the water may evaporate from the open ends found on the radiator cores and in the water tank in the heating system).
- Please explain what heat is.
- Heat is the energy transferred from a hot object to a cold object when two objects are in interaction.
- What is evaporation?
- This is the phenomenon in which liquids are heated and are converted to gas.
- Which laws in physics may be associated with heat and evaporation?
- Zeroth Law of Thermodynamics
- Heat exchange
- What are the physical quantities related to water evaporating?
- Temperature
- Specific heat
- Heat of evaporation
- What should we know/learn?
- Zeroth Law of Thermodynamics: If A and B systems are in thermal balance, A system is in thermal balance with system C, which is in thermal balance, and C system is in thermal balance with B system. This state of equilibrium is defined as temperature.
- The heat obtained from two interacting systems is equal to the heat emitted.
SESSION 2
Part 1The technical service personnel convey the problem at Mr. Şahin's house to the company engineer. The company engineer reviews the technical information about the heating system. According to the catalog, the boiler's water capacity is 30 liters and when fuel is consumed, a 25000 kcal of heat energy is obtained. The heat productivity of the heating system is 70%. This energy causes the water circulating in the radiators to heat up. The company engineer makes a calculation based on the supposition that all the water in the boiler evaporates and figures out how much energy must be produced and in what time this must happen.
- If we say that the average temperature of our city in the winter months is approximately 10oC, let's calculate the energy that's needed for the water to evaporate completely. (Make the density of water: 1 g/cm3 and Heat of evaporation: 540 cal/g)
The calculations show that the tank has 30 liters of water. If we say that the average temperature of our city in the winter months is approximately 10oC, let's calculate the energy that's needed for the water to evaporate.
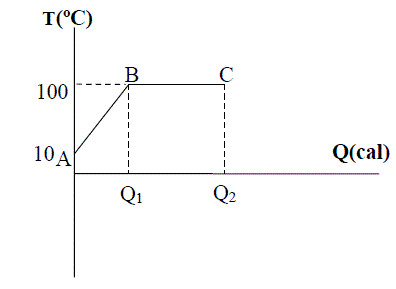
Boiling point: The temperature at the point at which a liquid's steam pressure is equal to atmospheric pressure is called its boiling point. The transition of the liquid to the gas phase at this temperature is called boiling; the opposite, that is, the transition from the gas phase to the liquid phase, is called condensation.
The heat required for the transition from A to B:
Q=m·c·ΔTQ: the heat required for the water to reach the temperature 100oC
m: mass
c: specific heat
ΔT: temperature change
Since the average temperature in our city in the winter months is 10oC and because the temperature of the water in the heating system will be equal to this when the system is not turned on, we first have to heat the water to boiling point. Then we have to calculate the energy that will be needed for the water to evaporate. We first have to find the energy needed for the transition from A to B and then from B to C and finally, we add this all up and find the energy we need to calculate
The heat required for the transition from B to C:
Q=m·Lbm: mass
Lb: Heat of evaporation
Q: Heat needed for water to change the gas state
Specific heat: This is the amount of heat per unit mass required to raise the temperature of matter 1oC. This changes according to the type of matter. This is a differentiating property of matter.
Temperature change: The difference between the first and last measured temperature.
Heat of evaporation: This is the energy required for a unit mass of matter that has come to boiling point to evaporate.
For water;
Specific heat: 1 cal/g·oC
Heat of evaporation: 540 cal·/gWe found in the review of the situation that there was 30 liters of water in the heating system. Since 1 liter is 1 dm3, 30 liters is 30 dm3. If we use what we learned before, we can say that m=V.d (m: mass; V: volume d: density) Water density:1 g/cm3 1 dm3=1000 cm3
30 dm3=3·104 cm3
m=30000 cm3·1 g/cm3
m=3·104 g is the density of this water
Since the water will be approximately 10oC, the water will first pass through A-B in the graph above before it reaches the boiling point, and the energy needed for this is Q1.
Since water boils at 100oC, the temperature change is:
ΔT = 100 - 10
ΔT = 90oCQ1 = QAB = m·c·ΔT
`Q_(AB) = 3*10^4 g.l(cal)/(g*^oC)*90^oC`
QAB = 27·105 cal
The interval at which the water coming to boiling point evaporates is the interval B - C on the graph. The energy necessary for this (Q2 – Q1);
Q2 – Q1=QBC = m·Lb
`Q_(BC) = 3*10^4 g*540(cal)/(g)`
QBC = 162·105 calThe energy needed for the water to evaporate:
Q = QAB + QBC
= 27·105 cal+ 162·105cal
=189·105 cal
- How much energy is transferred to the heating system when it is turned on and where does the remaining energy go?
We had learned that the heating system supplies 25000 kcal of energy and that 70% of this energy is spent on the water. (1kcal=1000cal, 25000kcal=25×106cal and 70% of this value is transferred to the water. This is 25·106 × 0.70= 17.5·106cal.
In real life, there are other materials in the system besides water (iron pipes, the metal and plastic components in the boiler). These materials need some energy to heat up as well.
- Let's calculate the amount of time needed for the heating system to make the mass of water evaporate.
We had learned that the heating system supplies 25000 kcal of energy and that 70% of this energy is spent on the water. The heating system supplies 25000 kcal of energy in an hour. We just calculated that 189·105cal is required for the evaporation of all of the water in the heating system.
(1kcal=1000cal, 25000kcal=25×106cal and 70% of this value is 17.5×106cal.
Therefore, the time needed for the heat system to supply this much energy is:
`t = (189*10^5)/(17.5*10^6)` t = 1.08 hours
t `~=` 65 minutes
- Interpret the result found in item 2. (Reminder: The heating system was stopping 25 minutes after all of the water was depleted)
Our heating system was stopping 25 minutes after all of the water was depleted. We calculated the time needed for evaporation to be 65 minutes. Our calculations refuted the idea that the water could evaporate because the heating system was supplying too much energy since the time needed for the entire mass of water to evaporate is longer.
- Compare what you know now with your hypotheses.
- There might be a problem with the connections in the plumbing in the house. (eliminated)
- There might be a problem with the heating system (mechanical installations). (eliminated)
- The water in the boiler might have evaporated. (eliminated)
- There might be water seepage in the parts of the installation where the pipes are hidden.
SESSION 2
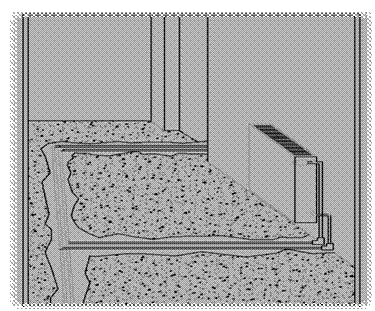
Part 2The calculations of the company engineer showed that all of the water in the boiler could not evaporate in the space of 25 minutes and that the problem stemmed from another mechanism; this was conveyed to Mr. Şahin. The engineer said that there could be a problem in the plumbing infrastructure (in the hidden pipes) and that the water seepage could be coming from there. The heating system is turned on once again and the same problem occurs. When the water in the boiler is depleted, the water continues to fill up the tank. At this point, the neighbor downstairs complains. The complaint is that there is water dripping down from the living room ceiling. It is therefore understood that the seepage is in the pipes in the living room. An estimate is made as to from where in the living room the water may be dripping and Mr. Şahin has the repairmen pull up the flooring in the living room (see Figure 2) and the leak is found. It is seen from an examination of the area that a nail had been nailed into one of the pipes in the plumbing at the time the floor covering was laid out.
Figure 2. The pipes in the living room
- What made the neighbor complain?
The neighbor complains when water starts dripping down from the ceiling after the heater is turned on and water is added.
- In line with the information given in the text above, what do you think the reason could be for the water loss that came about after the heater was turned on?
The water loss could be caused by a problem in the lower installation of the heating system. The loss could be because of a problem in the installation pipes.
- Which concept, principle or law in physics is the answer you gave to item 2 related to?
Heat, temperature, expansion
SESSION 2
Part 3Even when the heater is not turned on, there is still some water in the pipes in the infrastructure of the heater. This shows that when the heater is not on, there is no water loss in the boiler and that the water loss occurs when the heater begins to operate. It is thought that after the heater is turned on, it is the expansion in the iron nails and plastic pipes that causes the water loss (the loss in the boiler).
- What is expansion?
When objects are exposed to heat, their molecules start to move faster and the distance between them increases. As a result, the object expands, or in other words, its volume increases.
All expansions are actually increases in volume. When a long iron rod is heated, it lengthens and its thickness increases as well. Since the increase in its thickness, however, is negligible compared to the growth in its length, this expansion is described as only "lengthening." The same thing happens in surface expansion. When the surface of a metal sheet expands, this expansion is much greater than the increase in its thickness, and therefore the expansion in the thickness is neglected. This is called surface expansion.
- Explain in detail expansion in solids (with the necessary theoretical information and formulas).
Expansion in solids:
Because a heated solid material will expand in all three dimensions, expansion in solids occurs in the following way:
- Linear expansion
- Area expansion
- Volume expansion
- Linear Expansion:
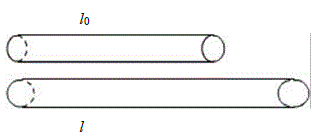
Let lo be the length of a metal rod before it is heated. When we heat the metal rod, it will grow longer and reach its final length of l. The change in length will be Δl= l - lo
lo= original length of the metal
λ = the linear expansion coefficient of the metal
ΔT = Tson - Tilk = the change in the temperature of the metal
Δl = l - lo = lo·λ·ΔT- Area Expansion:
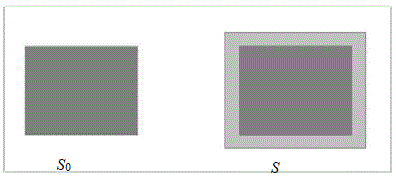
Let So be the surface of a metal sheet before it is heated. When we heat this metal sheet, the surface will increase and become its final surface S . The area expansion change is ΔS= S -So
So =the original surface area of the metal
2λ =the area thermal expansion coefficient
ΔT =the change in the temperature of the metal
ΔS = So·2λ·ΔT
- Volume Expansion:
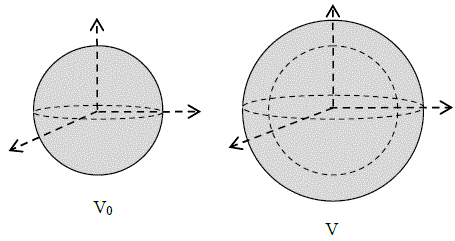
Let Vo be the original volume of a metal sphere before heating. When we heat the metal sphere, its final volume is V. The change in volume is ΔV=V-Vo
Vo =the original volume of the metal
3λ =volumetric thermal expansion coefficient of the metal sphere
ΔT =the change in temperature; where,
ΔV = Vo·3λ·ΔT
- Think about how the nail looks imbedded in the lower plumbing pipe and review this in the light of the principle of solids expanding.
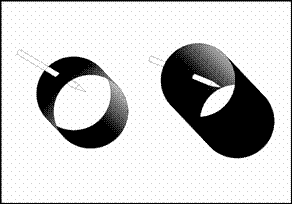
Figure 3. How the nail in the plumbing pipe looks from two different angles
We found the place where the water had leaked. When we opened it up and took a look, we saw that a nail had been driven into one of the pipes in the plumbing. Different views of the nail driven into the pipe can be seen below. Let's solve the problem using our knowledge about expansion. The water seepage occurs after the system is heated up. In other words, the expansion of the embedded nail and the pipe with the increase in the heat is what leads to the water loss. If we were to simplify this occurrence:
When we look very simply at the state of the nail as it looks in the figure, we can see the expansion in both the pipe and the nail.
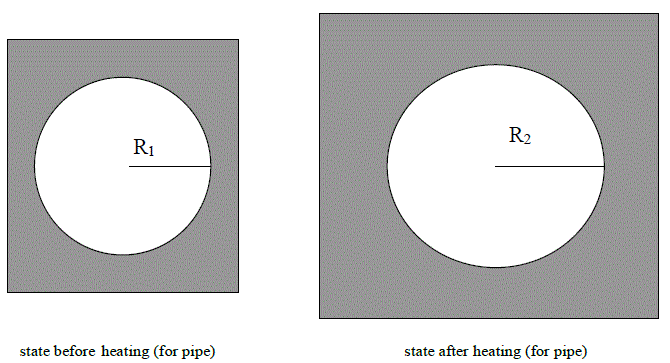
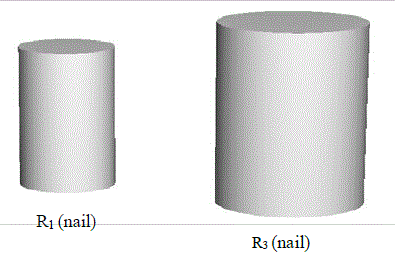
Before the heating system is turned on, as seen in the figure above, when the nail closes up the hole in the metal (since both have the same radius), there is no water seepage. When the heat is turned on, however, and the system starts heating up, the figures below (Radiuses R1 and R3) occur.
Since R2>R3, a space forms between the nail and the pipe and it is through this space that the water seepage occurs. The seeping water starts leaking down through the ceiling of the neighbor below.) occur.
- What must we learn?
- Learning that dimensions increase when matter is heated.
- Learning that dimensions change when temperatures of matter change.
- Learning about which properties of matter expansion depends on.
SESSION 3
FINALIt was found that the system stops automatically when the heat is turned on and a while later, the water in the heater is lost. It was found that the reason the water got depleted in the heater was because a nail had been driven into one of pipes in the plumbing. Because the temperature rises once the heater is turned on, both the nail and the pipe expand, and water then seeps between the pipe and the nail. It can be understood from this that different materials expand in different amounts. The area thermal expansion of the water pipe was more than in the nail (the heat expansion coefficient of plastic materials is about 10 times more than that of metals).
After finding where the water leaked from, the repairmen removed the nail from the pipe it had been embedded in. The leak in the perforated pipe that allowed the pipe to expand was repaired with a repair solution. After this repair was completed, there was no other place for the water in the heater to leak from. Mr. Şahin turned on the heater again and the heater continued to work even hours later. The heater was now operating and problem-free. So the problem with the heater in Mr. Şahin's house had been solved.
Can you now write up a one-session short scenario based on the inspiration the one above has given you?