Asia-Pacific Forum on Science Learning and Teaching, Volume 16, Issue 2, Article 7 (Dec., 2015) |
Evaluation of evidence: contributions to the enhancement of critical thinking
In this paper, critical thinking is understood as “a cognitive activity, associated with using the mind. Learning to think in critically analytical and evaluative ways means using mental processes such as attention, categorization, selection, and judgement” (Cottrell, 2005, p. 1). Barak, Ben-Chaim and Zoller (2007) suggest that there are several notable advantages of science education for promoting critical thinking. In fact, they consider that this ability is decisive for citizens in modern life. Similarly, Kuhn (2005) asserts that the modern world is characterized by technical and social complexities. This panorama has prompted a heightened interest in critical thinking in certain education programs in Australia (e.g. ACARA, 2012), Canada (e.g. CMEC, 1997), China (e.g. Bing & Thomas, 2006; Leung, 1991; Lewin, 1987), Colombia (e.g. MEN, 2006), England (e.g. NCE, 2014), France (e.g. MENESE, 2012), Japan (e.g. MEXT, 2000), Spain (e.g. MEC, 2007) and the United States (e.g. AAAS, 1993; NGSS, 2013; NRC, 2012; Yager & Brunkhorst, 2014).
Critical thinking is a fundamental skill for students of the 21st century (Choi, Ko & Lee, 2015). In science education, the need for promoting this kind of thinking is largely justified by studies that have confirmed most of its potentialities. Some of these are:
- The stimulation of students’ curiosity, interest and motivation (Kogut, 1996)
- The promotion of effective logical thinking among students (Koray & Köksal, 2009)
- The improvement of students’ comprehension of the nature of science (Montgomery, 2009)
- The betterment of learners’ conceptual understanding (Kogut, 1996; Zoller & Pushkin, 2007)
- The enrichment of learners’ argumentation and evidence evaluation (Chang, 2007; Chang & Rundgren, 2010; Jiménez-Aleixandre & Puig, 2012; Pallant & Lee, 2015)
As mentioned earlier, this paper focuses on the relevance of evaluating evidence for enriching critical thinking in the chemistry classroom. At this point, it is important to clarify that “thinking critically does not mean questioning all data, evidence and experts, but rather developing criteria for evaluating them” (Jiménez-Aleixandre & Puig, 2012, p. 1012, italics added). Indeed, the fact that students evaluate evidence does not necessarily mean that they use it to argue in chemistry class. The use of evidence is another goal that is not discussed in this paper.
On the one hand, the evaluation of evidence is a cognitive process requiring class activities and laboratory work that have been specifically designed to this end. Nonetheless, some chemistry teachers have difficulty in coming up with evidence evaluation scenarios in the classroom (Barak, Ben-Chaim & Zoller, 2007). The main reason for this is that some teacher training programs do not teach future teachers to promote thinking abilities (Archila, 2014ab; Xie & So, 2012; Zhou et al., 2012). The situation is all the more complicated when some school teachers are forced to spend more time in the classroom, leaving them with much less time for other activities that are also important in the teaching and learning process (e.g. learning assessment, exchanging viewpoints with other teachers and parents, lesson preparation, reading students’ production). This last has been strongly emphasized by Sahlberg (2010):
Although […] teachers’ work consists primarily of classroom teaching, many of their duties lay outside of class. […] in Finland […] teachers devote less time to teaching than do teachers in many other nations. For example, a typical middle school teacher in Finland teaches just less than 600 hours annually, corresponding to about four 45-minute lessons a day. In the United States, by contrast, a teacher at the same level devotes 1,080 hours to teaching over 180 school days […]. This, however, does not imply that teachers in Finland work less than they do elsewhere. An important—and still voluntary—part of […] teachers’ work is devoted to the improvement of classroom practice, the school as a whole, and work with the community. Because […] teachers take on significant responsibility for curriculum and assessment, as well as experimentation with and improvement of teaching methods, some of the most important aspects of their work are conducted outside of classrooms (p. 7, italics added).
Teachers’ actions should be regarded as crucial to the promotion of evidence evaluation (Jirout & Klahr, 2012; Koray & Köksal, 2009; Morris et al., 2012; Yenice, 2012). Nevertheless, one must bear in mind that the identity and the attributes shown by teachers in their practice are a complex construct, essential for encouraging professional development (Aristizábal, 2014; Enyedy, Goldberg & Welsh, 2006; Nogueira, 2014). These considerations would be highly relevant to initiatives in curriculum reform (Hernández, 2014).
On the other hand, Gott and Duggan (2003), Jiménez-Aleixandre and Puig (2012), Judge, Jones and McCreery (2009), Pallant and Lee (2015), and Yun and Kim (2015) emphasize that the evaluation of evidence can help individuals elaborate and communicate their own conceptions, opinions and postures. According to Gott and Duggan (2003), citizens are confronted by scientific evidence every single day; this might explain why it is imperative to offer learners opportunities to evaluate evidence in the chemistry classroom. Evidence evaluation is certainly useful for making decisions in issues such as climate change (Pallant & Lee, 2015), human cloning (Jiménez-Aleixandre & Puig, 2012) and methods of birth control (Gott & Duggan, 2003). In addition, Montgomery (2009) reports that evidence evaluation may assist in the development of a more informed understanding of the nature of science.In this study, two types of evidence are discussed: (i) experimentation in science and (ii) scientific communication. The evidence that is produced during experiments helps chemists to better understand chemical phenomena (Lehman & Bensaude-Vincent, 2007). Additionally, scientists need to communicate their investigations. The existence of papers or books can be understood as evidence of scientific communication (Nielsen, 2013). Indeed, “there is an international push to improve the effectiveness with which scientists communicate” (Mercer-Mapstone & Kuchel, 2015, p. 1614).
The use of historical controversies as an educational tool in science
As mentioned in the introduction, this study concerns the use of a historical chemical controversy to promote students’ assessment of evidence. This section presents an overview of past and current research involving this type of controversy. It is important to keep in mind that:
If we wish to use the history of science to influence [enrich] students’ understanding of science, we must include significant amounts of historical material and treat that material in ways which illuminate particular characteristics of science (Russell, 1981, p. 56).
This statement forms the basis of several studies that have explored the possible contributions of the history and philosophy of science (HPS) to the enhancement of learners’ understanding of the nature of science (e.g. Abd-El-Khalick, 2013; Allchin, Andersen & Nielsen, 2014; Matthews, 1994). Some authors have called for further research to examine the conditions and impact of a combined approach between HPS and thinking abilities for facilitating argumentation (Adúriz-Bravo, 2014) and critical thinking (Montgomery, 2009). This call confirms that “HPS is being shown to be also relevant to problems in the learning of the sciences” (Matthews, 1994, p. 208).
The literature on HPS and chemistry teaching has continued to expand in recent years (e.g. Garritz, 2013; Greca & Freire, 2014; Niaz & Rodríguez, 2000). Some studies show efforts to place HPS on the curriculum (Monk & Osborne, 1997; Welch, 1979) and to explore the advantages for chemical education (Izquierdo, 2013). There is increasing research interest in the use of historical controversies in science education (Archila, 2015; de Hosson, 2011; Montgomery, 2009; Niaz, 2000, 2009). There is also a prevalent consensus on the need for more investigations that would provide empirical data for examining and expanding the scope of this type of controversy.
The historical content often included in science textbooks (Russell, 1981) and chemistry textbooks (Niaz & Coştu, 2013) needs to be dramatically improved. A historical and philosophical view of chemistry shows the existence of controversies as a habitual part of the daily work of a chemist (Garritz, 2013; Greca & Freire, 2014). Tsaparlis and Finlayson (2014) recognize that the use of historical chemical controversies is imperative in chemical education. Indeed, controversies in science are useful for helping students to understand how scientists actually work (Silverman, 1992). Hence, historical chemical controversies could offer opportunities for students to enrich their learning of chemistry and their learning about chemistry. Some examples of historical chemical controversies that have been used in education are listed below:
- Controversy between Jöns Jacob Berzelius’s (1779–1848) and Auguste Laurent’s (1807–1853) postulates over the substitution phenomenon (Archila, 2014c)
- Edward Turner’s (1796–1837) postulate and the atomic weight controversy (Campbell, 1981)
- Controversy between the European school lead by Svante August Arrhenius (1859–1927) and the British school lead by Henry Edward Armstrong (1848–1937) over the dissociation phenomenon (de Berg, 2014)
- Controversy surrounding quantum mechanics and quantum chemistry (Garritz, 2013)
- Controversy between Robert Andrews Millikan (1868–1953) and Felix Ehrenhaft (1879–1952) surrounding “The oil drop experiment” (Niaz, 2000; Niaz & Rodríguez, 2005)
It is important to clarify that promoting evidence evaluation has not been the objective behind the inclusion of the aforementioned examples in textbooks. This is where the originality and authenticity of the present study come in. Our paper addresses the controversy between the chemists Carl Wilhelm Scheele (1742–1786), Joseph Priestley (1733–1804) and Antoine Laurent de Lavoisier (1743–1794) surrounding the question, “Who discovered oxygen?”. This controversy is recreated in the play “Oxygen” written by Carl Djerassi and Nobel laureate Roald Hoffmann (2001a). “The ethical issues around priority and discovery at the heart of this play are as timely today as they were in 1777” (Djerassi & Hoffmann 2001b, p. 5). Hence, in this investigation drama is used as a learning strategy in science education (Braund, 2015; Klassen & Froese Klassen, 2014; Ødegaard, 2003; Pongsophon, Yutakom & Boujaoude, 2010). In addition, “drama is meant to be a very powerful teaching strategy for enhancing meaningful learning in science” (de Hosson & Kaminski, 2007, p. 622).
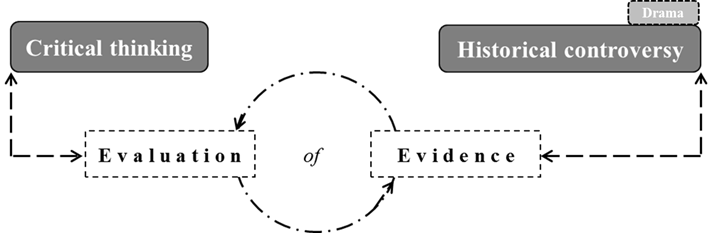
The literature review presented in this section is what inspired the combined HPS–critical thinking approach proposed in this study (Figure 1). The premise of this investigation is that a historical chemical controversy can provide students with evidence for evaluation. As mentioned earlier, evidence evaluation promotes critical thinking (Jiménez-Aleixandre & Puig, 2012) and a more informed understanding of the nature of science (Montgomery, 2009).
Figure 1. Combined approach between critical thinking and HPS.